Flamingo Tongue
Cyphoma gibbosum

Taxonomy
Kingdom Animalia
Phylum Mollusca
Class Gastropoda
Order Neotaenioglossa
Family Ovulidae
Common name: Flamingo Tongue
Average size: 0.75 to 1in. (1.8 to 3 cm)
Depth: 6 – 45 ft (2-15m)
Longevity: at least 2 years
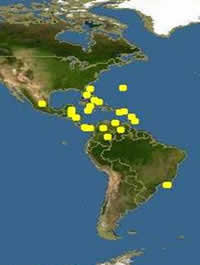
Distribution: Aruba, Belize, Bonaire, Caribbean Sea, San Andres, Cayman Islands, Jamaica, Colombia, Costa Rica, Cuba, Curaçao, Lesser Antilles, Mexico, Panama, Puerto Rico and Venezuela.
Habitat
 Cyphoma gibbosum, aka the Flamingo Tongue snail, is a marine gastropod that lives sub-tidally and is commonly found on many of the coral reefs of the Caribbean and the tropical western Atlantic. They exhibit a very unique and easily recognizable mantle which is heavily decorated with a distinctive irregularly-shaped orange, white and black spotted pattern. The Flamingo Tongue Snails are members of the phylum Mollusca, which also contains fellow invertebrates the octopus and oysters. The class Gastropoda is reserved for marine snails with and without shells or those who just possess a reduced shell. Although C. gibbosum have few natural predators, hogfishes, spiny lobsters and some pufferfishes were found to be some of their main predators (Nahabedian et al, 2007).
Cyphoma gibbosum, aka the Flamingo Tongue snail, is a marine gastropod that lives sub-tidally and is commonly found on many of the coral reefs of the Caribbean and the tropical western Atlantic. They exhibit a very unique and easily recognizable mantle which is heavily decorated with a distinctive irregularly-shaped orange, white and black spotted pattern. The Flamingo Tongue Snails are members of the phylum Mollusca, which also contains fellow invertebrates the octopus and oysters. The class Gastropoda is reserved for marine snails with and without shells or those who just possess a reduced shell. Although C. gibbosum have few natural predators, hogfishes, spiny lobsters and some pufferfishes were found to be some of their main predators (Nahabedian et al, 2007).
Behaviors
This gastropod mollusk is a predator of the gorgonian corals and is typically found on sea whips, sea fans, sea rods, and sea plums (Oceana 2010). They specialize in consuming the toxic flesh of gorgonian corals by utilizing their unique feeding appendage, the radula. This belt sander-like tongue structure is exclusive to the Mollusks and it is used to scrape away the coral tissue so nutrients can be absorbed. Due to their indifferent feeding strategy, only segments of a bare-naked gorgonian coral skeleton is left behind (Beautiful Oceans, 2011); however, the silver lining here is that the coral will slowly start to undergo regeneration to replace the affected areas. Flamingo tongues are smart enough to know not to graze their host gorgonian to death; instead, they transfer hosts periodically by crossing over to the next coral (Oceana 2010).
The soft tissue of these gorgonian corals contains a toxic chemical and when the C.gibbosum feeds, not only do they not suffer any harm, but they actually incorporate these secondary chemicals and becomes toxic themselves. As a direct result, the chemicals yield a distasteful experience to predators when they try to feed on the snails. These colorful mantle markings are used as warning signals to potential predators meaning “stay away.” When these kinds of warning colorations are used by organism, it is called aposematic coloration (EoL, 2011).
Even with an unpleasant taste, it seems that some predatory fishes will feed upon these snails. In 2007, Dr. Burkepile and Dr. Hay noticed that when large predatory fishes and invertebrates were excluded from certain areas of a coral reef located in the Florida Keys, the Flamingo Tongue Snail population density increased an astonishing 19-fold. Gorgonian corals experienced more frequent and much more extensive grazing damage (Burkepile and Hay, 2007). Therefore, it could be said that the predatory species of C. gibbosum appear to be actually indirectly protecting the corals and that if these predators became overfished, there would be devastating consequences to the gorgonian corals population (EoL, 2011).
Reproduction and Life Cycle
Since adults are dioecious and do not undergo a sex change, sexual reproduction occurs between adults when a male approaches a female and crawls up onto the right side of her shell. Here, the male extends a white tube under the edge of the female’s shell and begins mating. After up to 4 of hours of mating and four days, the female will lay encapsulated white translucent eggs onto the bare axis of gorgonians, which is exposed from the snail’s feedings. It is believed these areas are chosen since there is an absence of the gorgonian’s tissue toxins. Since each egg capsule contains multiple embryos (up to 300), a speckled appearance is demonstrated (Oceana, 2010). Even though oviposition occurs approximately during a lunar cycle, females have been known to lay several egg masses within a single cycle. After about ten days, the capsules hatch and the young free-swimming larvae disperse to live and feed on the plankton (Nahabedian et al, 2007). The larvae gradually metamorphose into juveniles, then adults.
Flamingo tongues activity levels increase when the sun starts to rise and while they actively feed on their host throughout the day. As the day ends and the sun sets, their activity levels decrease and they remain relatively inactive throughout the night as they settle on their gorgonian host (Oceana 2010).
Recent Research
There has been much research conducted over the recent years that focuses on the amount of host specificity, distribution and phylogenetic relationships between the Atlantic Ovulidae. Cyphoma Röding, is the dominant genus in the Caribbean with 14 different species of which Cyphoma gibhosum (Linnaeus, 1758) is the most common (Reijnen’ et al, 2010). The studies were concentrated off the leeward side of the western coast of Curaçao, Netherlands Antilles. Through the months of April – June in 2005, ovulid snails and as well as their octocoral hosts were sample and analyzed at 32 different areas to test for prey preference associated with C. gibbosum. Out of the total 104 ovulids that were collected, 72 represented Cyphoma gibbosum and were associated with 21 different alcyonacean species, representing nine genera (Reijnen’ et al, 2010). Their research also confirmed that there are indeed several Octocorallia groups that exist without any associated ovulids; they found approximately 20 within their concentrated area.
In 2003, the density and gorgonian host-occupation patterns of Cyphoma gibbosum was studied and concentrated in the Florida Keys. Two main objectives were focused on: the comparison between heavily fished areas and protected marine areas, and secondly, is the preferred gorgonian–host occupancy relationship between C. gibbosum proportional or disproportional (Chiappone et al, 2003). Interestingly enough, out of the collected 129 snails, only one snail was not found a gorgonian colony. Similarly, not a single snail was found in 40% of the shallow reef habitats that were studied; this confirms that a variable density was definitely observed. A significant difference in C. gibbosum frequency was also determined, especially in the areas of the lower Keys. There was a 3.9x greater calculated mean snail density between fished areas when compared protected areas (Chiappone et al, 2003). This same outcome was found in the Upper Keys area when fished areas were again compared to protected areas. This makes sense because if an area is highly fished, there is not as many predators available to prey upon the snails and keep their densities more maintained when compared to protected areas where there would be an increased number of predators available to feed on the snails. Out of the total 127 gorgonians that were examined, about 58% of C. gibbosum occurred in solitary and about 31% were found in pairs (Chiappone et al, 2003). An unexpected outcome and significant difference that was detected in the gorgonian host-occupation was that more coral from the Family Plexauridae was occupied versus coral from the Family Gorgoniidae (Chiappone et al, 2003). Lastly, most of the gorgonians were considered both occupied proportionally and under-occupied well other corals displayed a disproportional occupancy.
As previously mentioned, Burkepile and Hay in 2007 noticed that Cyphoma gibbosum increased to an mind blowing 19-fold when predatory fishes and invertebrates were barred from certain areas of the Florida Keys coral reef. Since these snails are the primary consumer of Gorgonians, damage rendered to these soft corals also increased by 8 fold. Even though Burkpile and Hay express that gorgonian damage due to these C. gibbosum grazings can be minimal, it was observed that about 75% of tissue was lost on a 0.8 m tall Eunicea calyculata, due to the C. gibbosum grazing, while the cages were in place. It can be concluded that the best environment for gorgonians to prosper are in areas that marine life are protected so that predatory animals can feed on the snails at will thus keeping their densities in control.
Commercial Importance
 Since the Flamingo tongue’s unique colorful shells are recognized worldwide, they are becoming overly collected. Many snorkelers and scuba divers that come across these snails make the mistake of thinking that the brightly colored spots are on the shell are permanent. In fact, the color of the shell is only due to the mantle of the animal inside the shell and when the animal dies, the bright color pattern does with it, leaving only an apricot colored shell (Nahabedian et al, 2007). Due to their unique color patterns, flamingo tongue have become a staple in shell collections and many businesses even specialize in selling of these shells to produce popular jewelry that is sold world- wide. Even though they are not currently on the IUCN endangered species list yet (Oceana, 2010), due to their increased popularity, C. gibbosum populations are in rapid decline (Nahabedian et al., 2007) and protective measures should be taken.
Since the Flamingo tongue’s unique colorful shells are recognized worldwide, they are becoming overly collected. Many snorkelers and scuba divers that come across these snails make the mistake of thinking that the brightly colored spots are on the shell are permanent. In fact, the color of the shell is only due to the mantle of the animal inside the shell and when the animal dies, the bright color pattern does with it, leaving only an apricot colored shell (Nahabedian et al, 2007). Due to their unique color patterns, flamingo tongue have become a staple in shell collections and many businesses even specialize in selling of these shells to produce popular jewelry that is sold world- wide. Even though they are not currently on the IUCN endangered species list yet (Oceana, 2010), due to their increased popularity, C. gibbosum populations are in rapid decline (Nahabedian et al., 2007) and protective measures should be taken.
Personal Interest
I am really glad that I picked this invertebrate for my research project because I enjoyed learning about this magnificent creature and its fascinating ways. To me, C. gibbosum has the best of both worlds; a quirky name and a one-of-a-kind look. I am just in awe of its random irregular-shaped markings in combination with its toxic flesh. I am really hoping that I came across many of these snails while I am down in Belize. It’s devastating to learn how over collected they have become from human predation. I hope that some type of protection measures are taken before it’s too late and the oceans lose such a truly intriguing creature.
Suggested Links
Flamingo Tongue Identification
Works Cited
- Reijnen, Bastian T., Bert W. Hoeksema, and Edmund Gittenberger'. "Host specificity and phylogenetic relationships among Atlantic Ovulidae (Mollusca: Gastropoda)." Contributions to Zoology 79.21 May (2010): 69-98. Web. 19 May 2011.
- Chiappone, Mark, Helga Dienes, Dione W. Swanson, and Steven Miller. "Density and Gorgonian Host-occupation Patterns by Flamingo Tongue." Caribbean Journal of Science 39.1 (2003): 116-27. Web. 24 May 2011
- Burkepile, Deron E., and Mak E. Hay. "Predator release of the gastropod Cyphoma gibbosum increases predation on gorgonian corals." Oecologia 154.1 Sept. (2007): 167-73. Print.
- Barna Páll-Gergely. Editor. "Cyphoma gibbosum (Linnaeus, 1758)". Encyclopedia of Life, available from "http://www.eol.org/pages/593724". Accessed 23 May 2011
- Nahabedian, Sarah, James B. Wood, and Melissa Parr. "Flamingo Tongue Snail (Cyphoma gibbosum)." Marine Invertebrates of Bermuda. Ed. Dr. James B. Wood. BBSR, 2007. Web. 21 May 2011.< http://www.thecephalopodpage.org/MarineInvertebrateZoology/Cyphomagibbosum1.html>.
- "Flamingo Tongue - Cyphoma gibbosum." Beautiful Oceans, n.d. Web. 24 May 2011.< http://www.beautifuloceans.com/creatures/a-m/flamingo-tongue/239-flamingo-tongue-cyphoma-gibbosum.html>.
- "Flamingo Tongue Cyphoma gibbosum." Oceana, North America. Oceana, 2010. Web. 20 May 2011.< http://na.oceana.org/en/explore/creatures/flamingo-tongue>.